R&D
WE WILL MAKE HEADWAY INTO THE GLOBAL MARKET BEYOND THE DOMESTIC MARKET BY GATHERING THE ABILITY OF JW
Technology
Small molecules
-
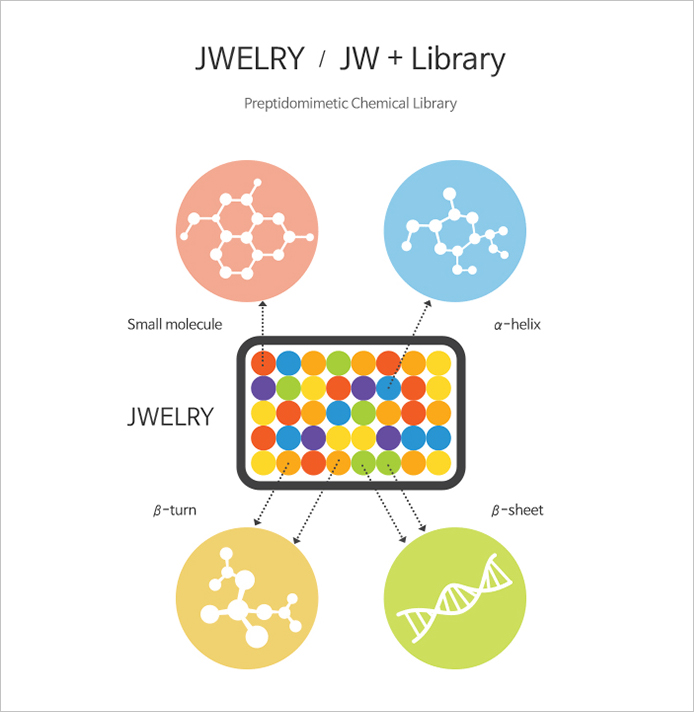
JWELRY
- Compound library composed by unique peptide mimetic compounds
- A unique Drug Discovery Platform composed of diverse cancer cell lines and their genetic information.
-
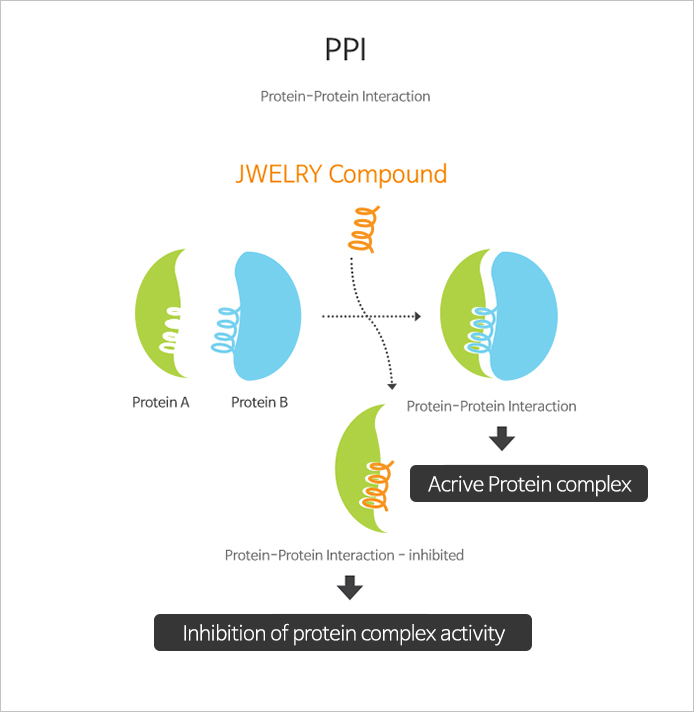
"JEWELRY" is an abbreviated word of JW excellent library. As a collection of unique peptide mimetic compounds possessed by JW. In addition to the usual synthetic small molecule chemical compounds, it contains about 25,000 peptide-like structural compounds that mimic 3D structures found in proteins, such as α-helix, β-turn, and β-sheet. With various screening systems (ex. HTS), JWELRY has enabled a discovery of innovative novel drug candidates that regulate protein-protein interaction critical in the pathology of cancer or immunological diseases.
-
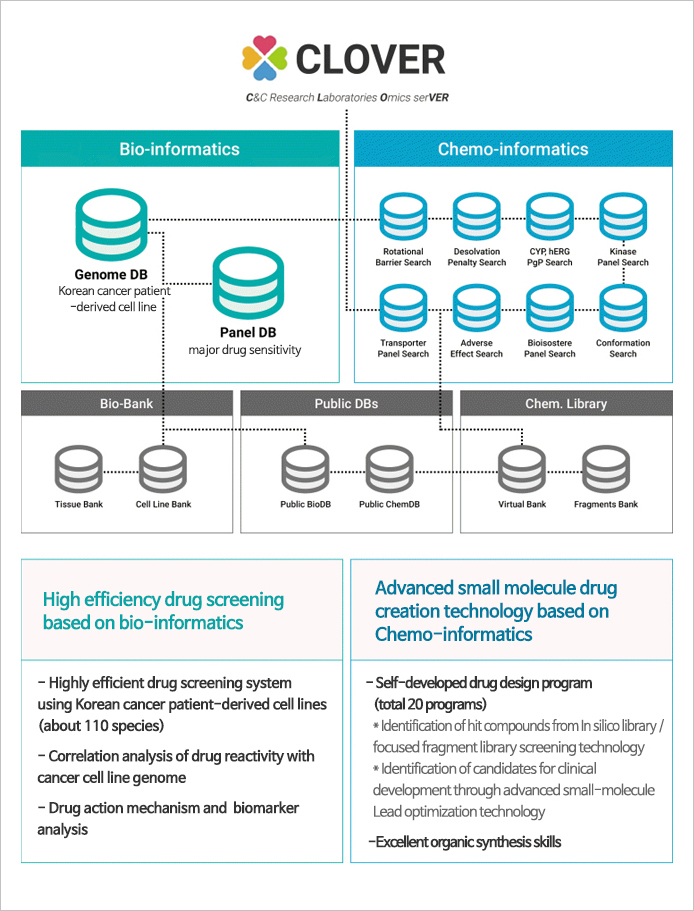
CLOVER
- Big data platform accumulating data of cancer cell lines, tissues, and genetic information, etc.
-
Immunotherapies
-
DC vaccine technology
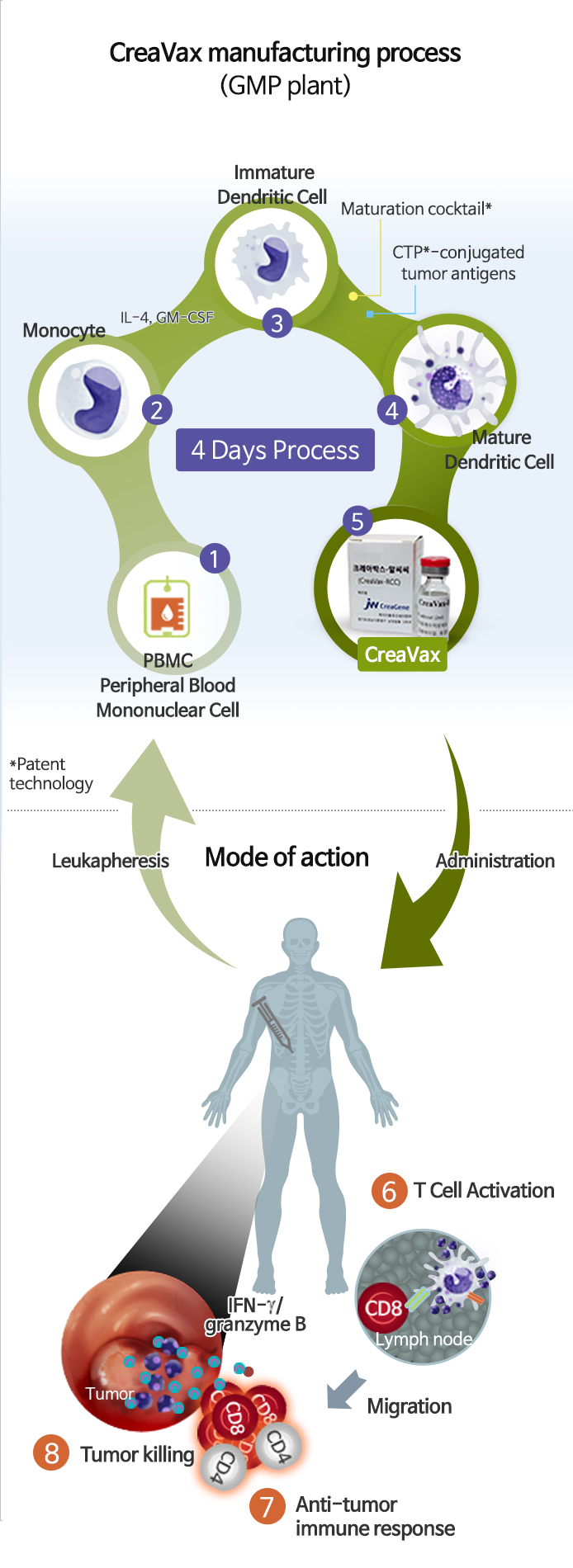
- DC vaccine derived from patient’s own blood cells is the personalized immunotherapeutic vaccine for cancer and auto-immune diseases. Disease specific antigens-loaded dendritic cell allows the immune cells to recognize and attack only cancer cells through a tumor specific antigen recognition process.
-
Dendritic cell (DC) is the representative antigen-presenting cells that act as command towers of the human immune system. DC exerts immune-surveillance for exogenous and endogenous pathogens and activates T lymphocytes giving rise to various immunological responses. Furthermore DC is involved in the pathophysiology of several diseases such as cancer, infectious disease, or autoimmune diseases, but they can also be used as therapeutic tools in these conditions.
CreaVax is the DC therapy brand name of JW CreaGene and generated from patient’s own monocytes in specialized method of JW CreaGene. When the enforced DCs are injected into the patient, they migrate to lymph node, educate T cells to recognize cancer cells, and induce activation and proliferation of T cells. Finally, the activated T cells move to tumor site to kill cancer cells. And released antigens from dying cancer cells can induce secondary immune responses. Furthermore, DC vaccination induces memory T cells and these T cells help to prevent cancer recurrence.
Publication
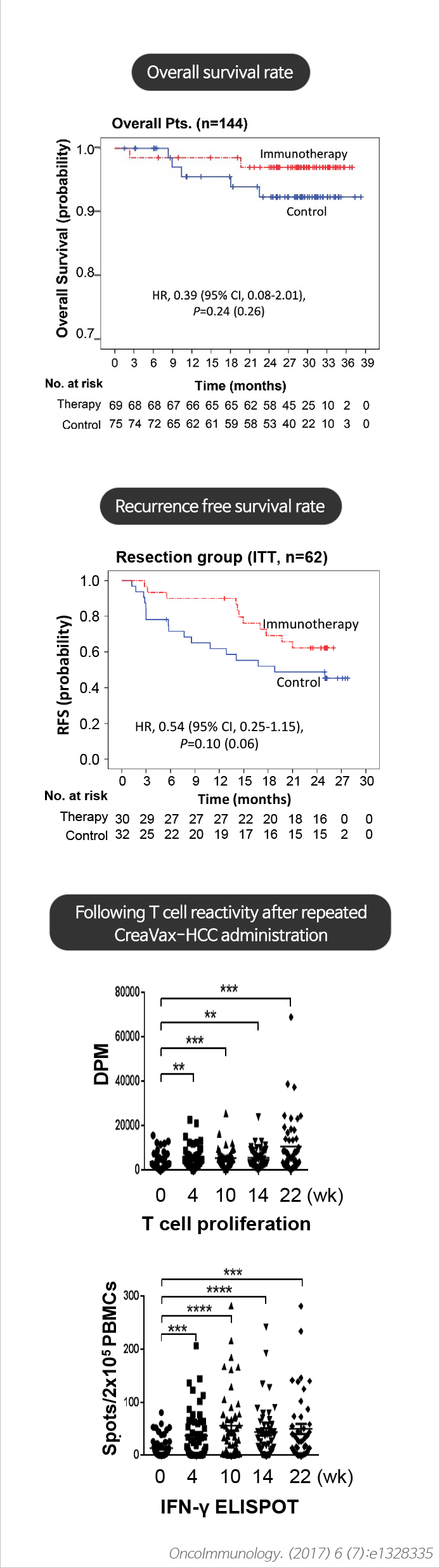
- Adjuvant Immunotherapy with Autologous Dendritic Cells for Hepatocellular Carcinoma, Randomized Phase II Study. OncoImmunology. 6(7):e1328335 (2017)
- A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br J Cancer. 113(12):1666-76 (2015)
- DC-Based Immunotherapy Combined with Low-Dose Methotrexate Effective in the Treatment of Advanced CIA in Mice. J Immunol Res. 2015: 834085 (2015)
- Dab2, a negative regulator of DC immunogenicity, is an attractive molecular target for DC-based immunotherapy OncoImmunology 2015 Feb 3;4(1):e984550 (2015)
- Dendritic cell-based therapeutic cancer vaccines: past, present and future Clin Exp Vaccine Res. 3(2):113-6 (2014)
- Egr2 induced during DC development acts as an intrinsic negative regulator of DC immunogenicity Eur J Immunol. 43(9):2484-96 (2013)
- Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma Int J Oncol. 2012 Nov;41(5):1601-9.
- Photodynamic therapy-mediated DC immunotherapy is highly effective for the inhibition of established solid tumors. Cancer Letters 2012 Nov;324(1):58-65.
- Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol 2012 Nov;41(5):1601-9.
- Photodynamic therapy-mediated DC immunotherapy is highly effective for the inhibition of established solid tumors. Cancer Lett. 2012 Nov 1;324(1):58-65.
- CISH is induced during DC development and regulates DC-mediated CTL activation. Eur.J. Immunol. 2012 Jan;42(1):58-68.
Excellent tolerability and recurrence suppression effect
Intellectual property
-
CTP Technology
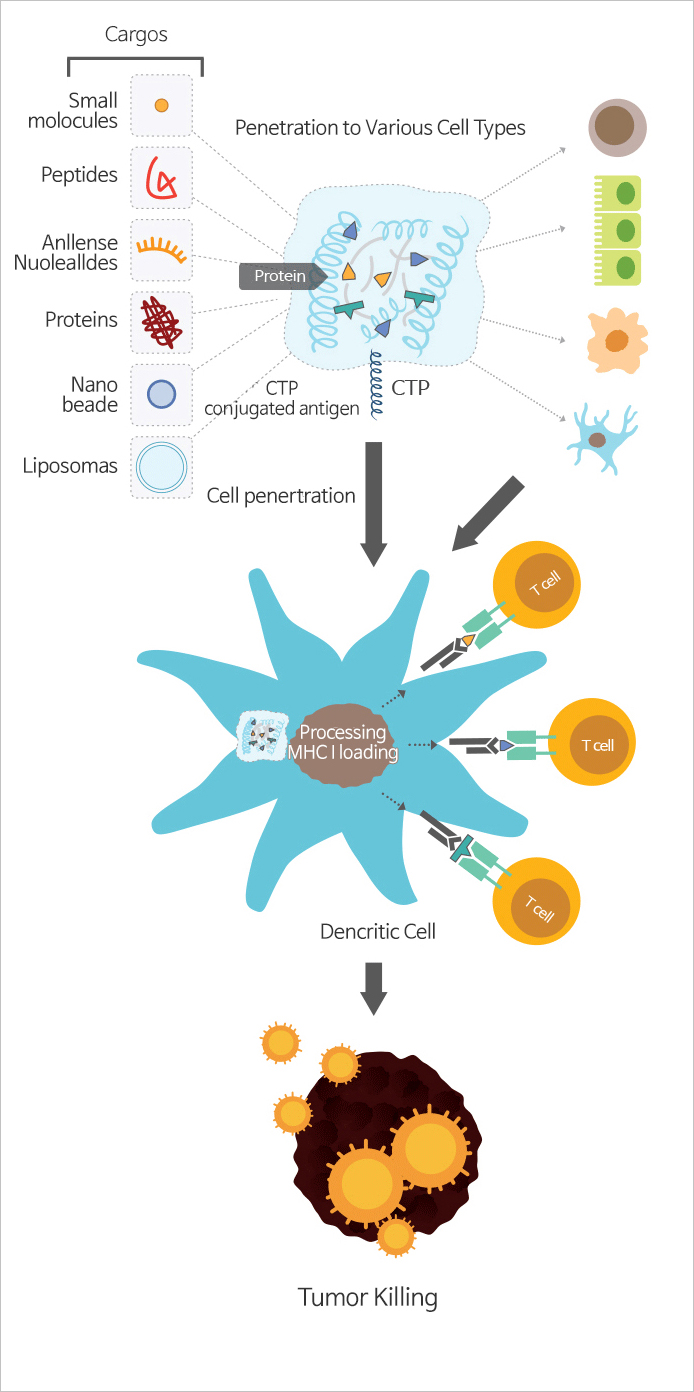
- CTP (Cytoplasmic Transduction Peptide) Technology is effective drug delivery system that ensure the cytoplasmic delivery of the CTP-fused polymeric biomolecules
-
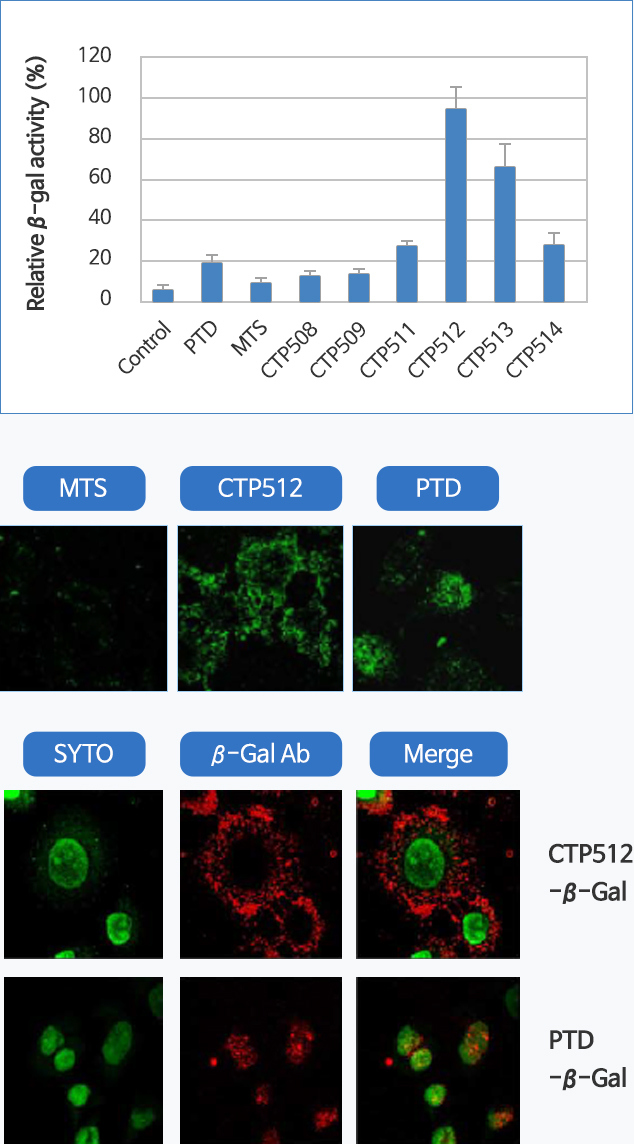
CTP-fused drug can display effective results even at low concentrations due to improved efficacy in cell membrane penetration. Our specialized antigen delivery technology is able to induce high level of sensitization effects and immune reactions on a diverse range of targets. The reason is because CTP-fused recombinant protein antigens induce cancer-specific immunity in dendritic cell treatment.
Publication
- Cytoplasmic transduction peptide (CTP): New approach for the delivery of biomolecules into cytoplasm in vitro and in vivo. Exp Cell Res. 312(8): 1277-88 (2006)
- Increased synthesis of hyaluronic acid by enhanced penetration of CTP-EGF recombinant in human keratinocytes. J Cosmet Dermatol. Jan 20. (2019)
Excellent cell permeability
Preference of cytoplasmic retention
Intellectual property
- IFN-α Fusion Protein Comprising Interferon-α Linked to Cytoplasmic Transduction Peptide and Polyethylene glycol
- IFN-α fusion protein comprising interferon-α and cytoplasmic transduction peptide
- Cytoplasmic Transduction Peptides and Uses thereof
Incrementally modified drugs (IMD)
-
SMEDDS
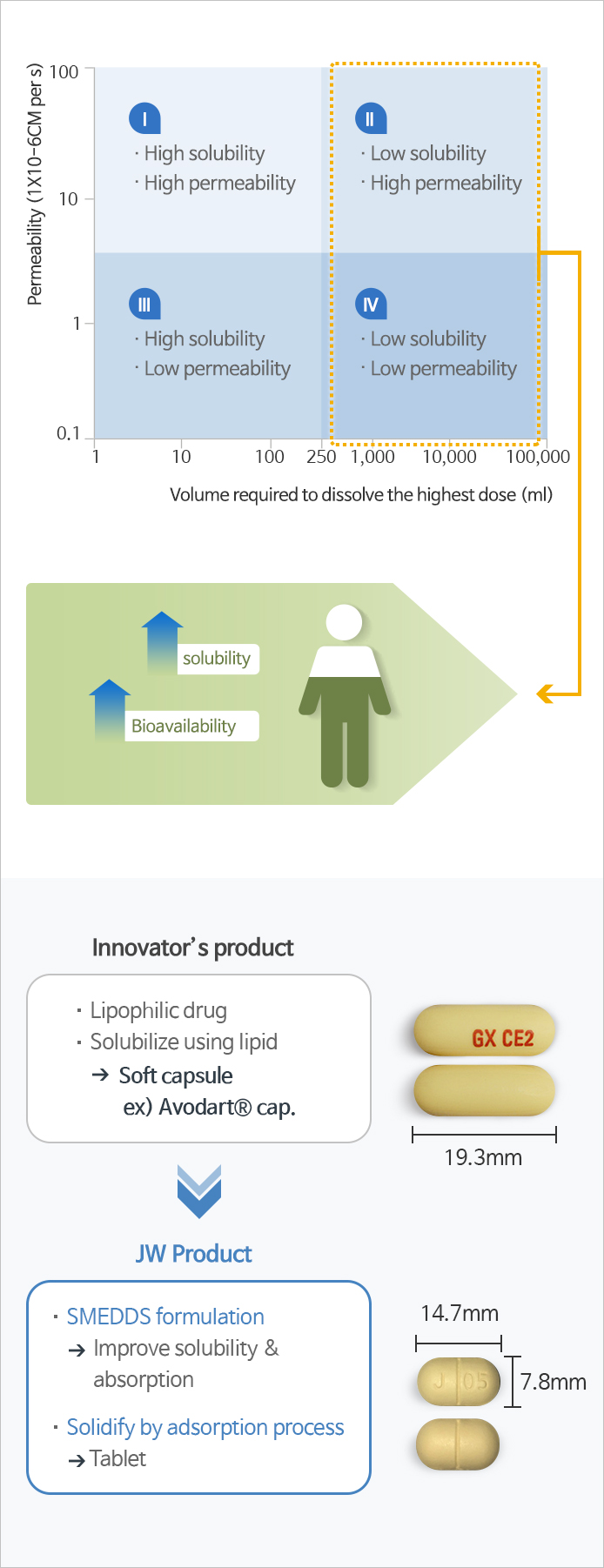
- Self-microemulsifying drug delivery system (SMEDDS), one of the solubilizing technologies for poorly soluble drugs, is a technology that improves the solubility and bioavailability of poorly soluble drugs by mixing surfactant, co-surfactant, oil, and the drug at the optimum ratio.
- JW Pharmaceutical's proprietary technology, which overcomes the shortcomings and limitations of existing poorly soluble drug formulations and improves the solubility and bioavailability of drugs to maximize drug safety and ease of administration.
-
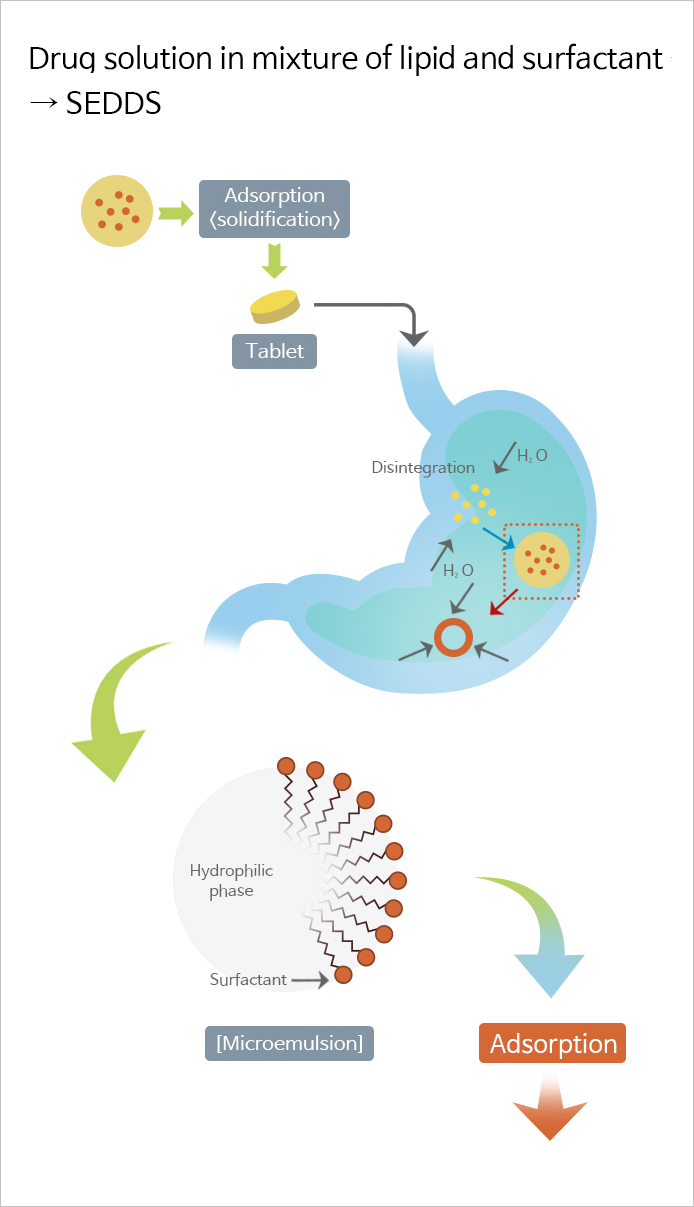
Self-microemulsifying drug delivery system (SMEDDS) is a solubilization technology for improving bioavailability of poorly soluble drugs. By combining surfactant and co-surfactant with a poorly soluble drug, this technology improves the bioavailability of the drug by forming fine emulsion droplets upon dilution with physiological fluid after administration.
This proprietary technology utilized SMEDDS technology to solubilize the poorly soluble drug, and further adsorbed the mixture to the solid carriers in order to prepare them into tablets, which enabled solidification of soft capsules into tablets and overcome the shortcomings of temperature-sensitive soft capsules and increase the convenience of administration.
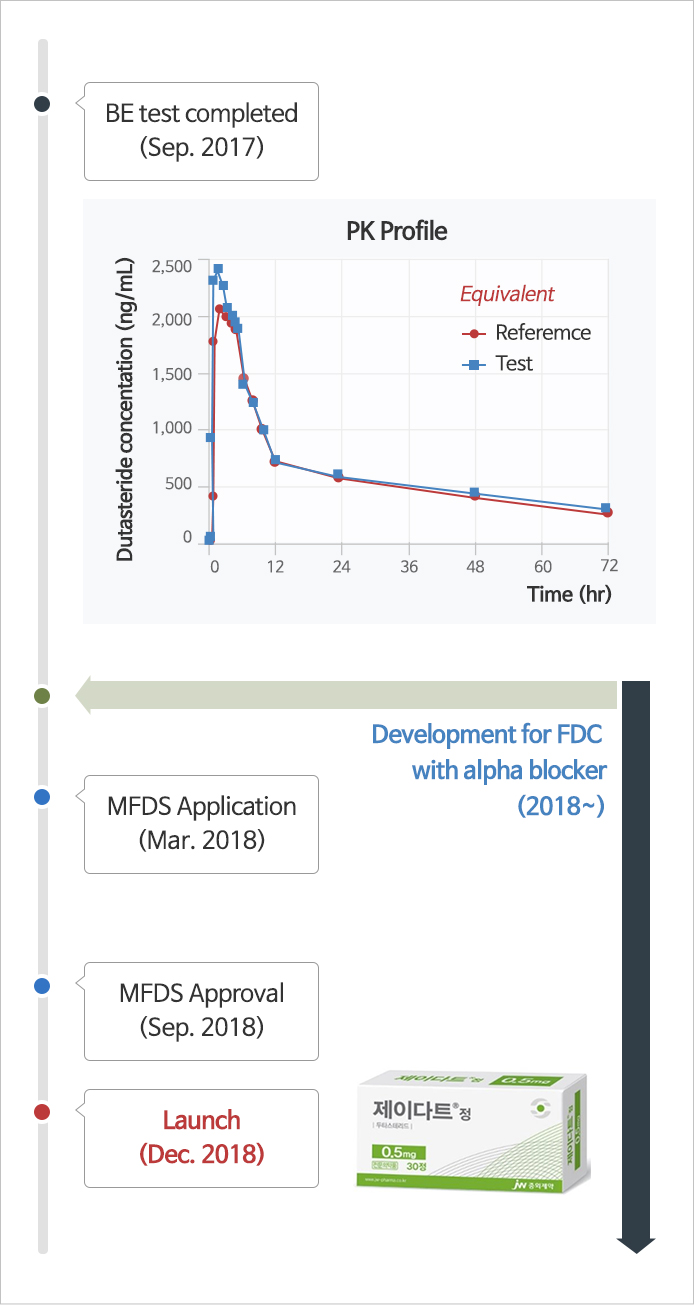
J-dart Tablets
Intellectual property
Title of the invention : Solid preparations containing dutasteride and preparation method
Application Date : 2017.09.01
Application number : 10-2017-0111953
Active pharmaceutical ingredient (API)
-
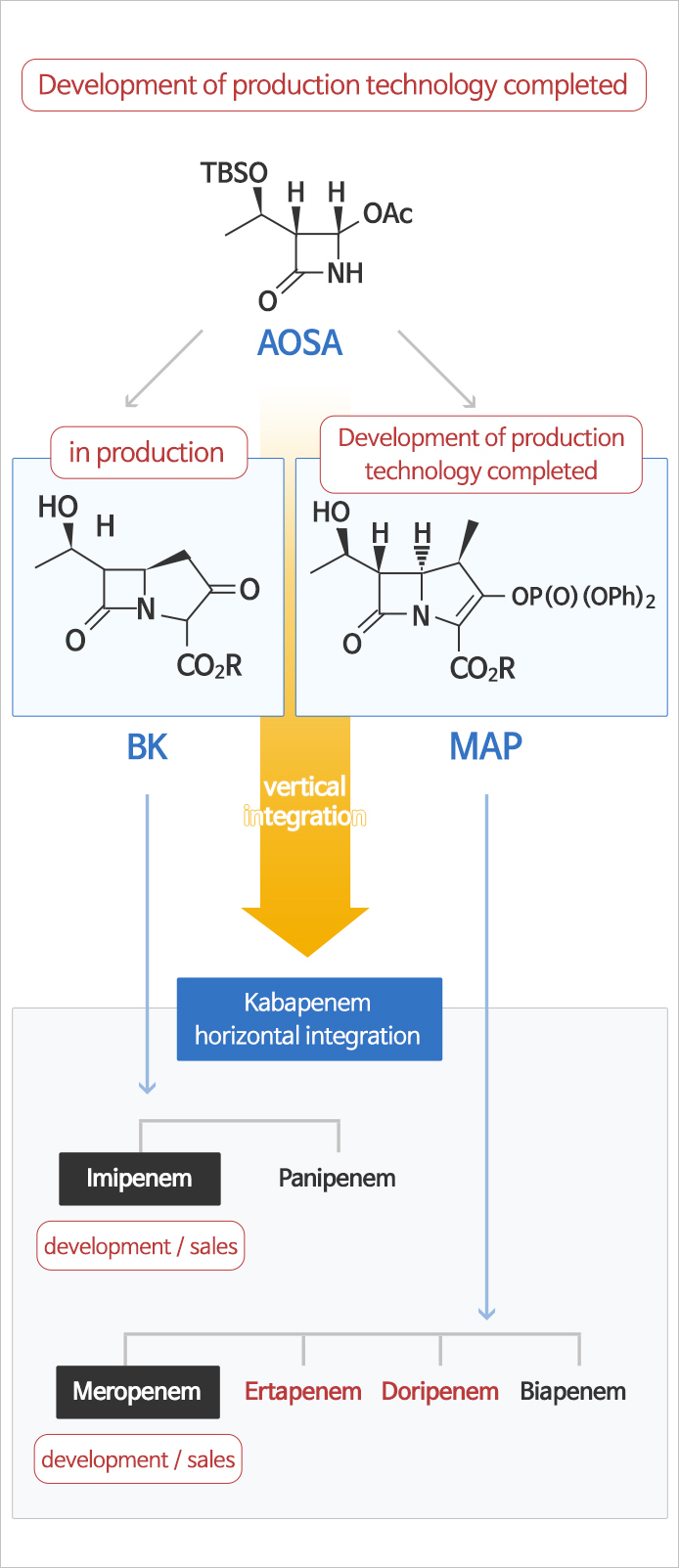
Carbapenem Manufacturing & Purification
- Imipenem, meropenem and doripenem have been commercialized through a study of systematic using AOSA, a key starting material of carbapenems antibiotic agents.
-
The core technology of carbapenem manufacturing is high pressure hydrogenation reaction and high quality Prep-HPLC purification.
-
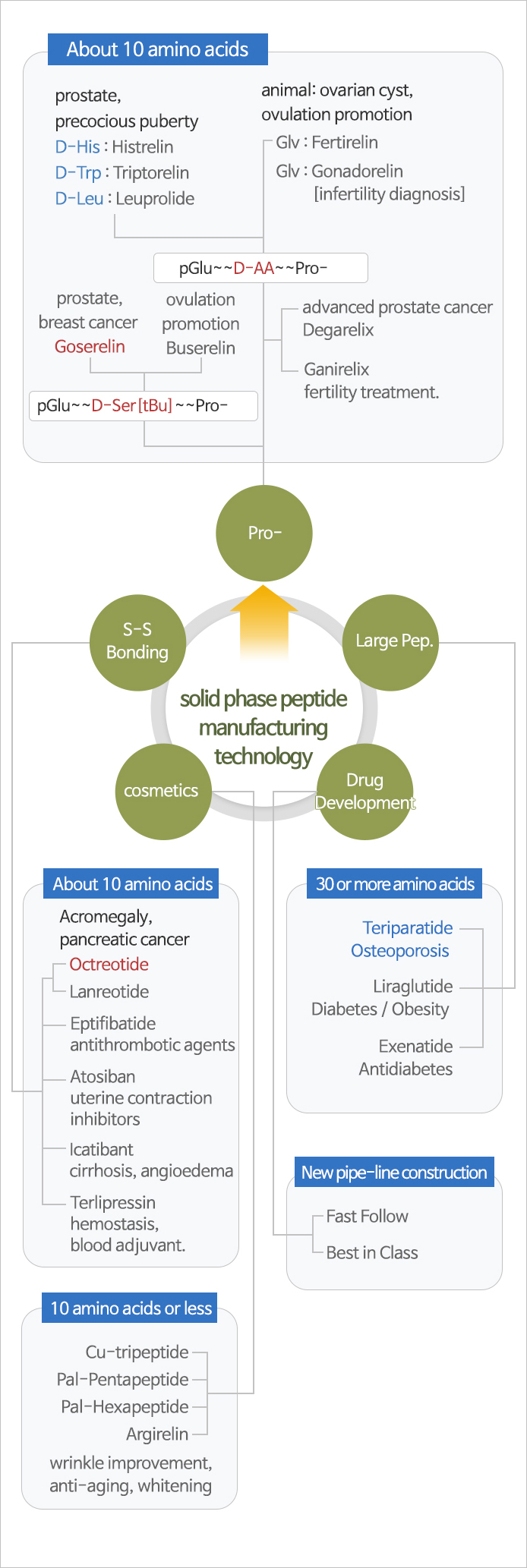
Solid Phase Peptide Synthesis
- Manufacturing Process was completed through SPPS(solid phase peptide synthesis) and Prep-HPLC/Resin purification technology about Goserelin and Octreotide.
-
After first amino acid was loaded on the resin, targeted peptide was synthesized through deprotection and amide coupling sequentially.
-
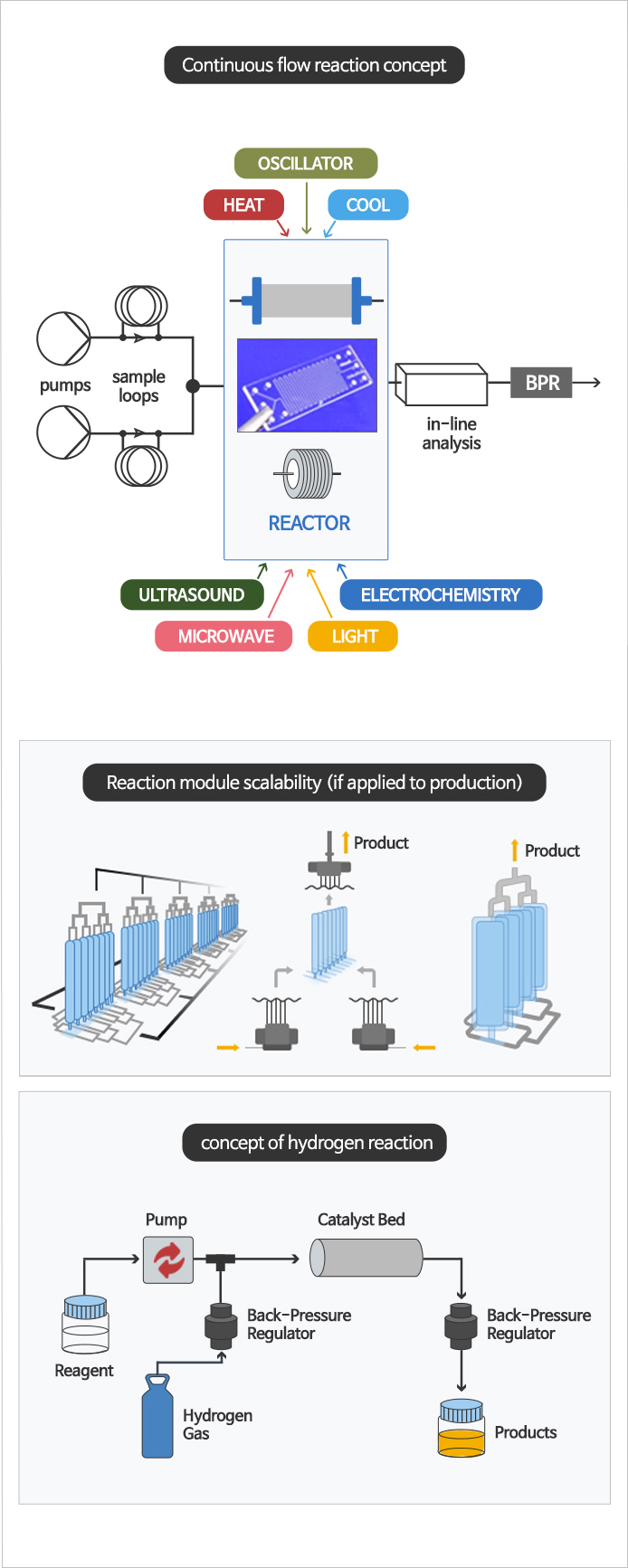
Continuous reaction, Flow chemistry reaction
- Flow chemistry or continuous flow chemistry begins with two or more streams of different reactants pumped at specific flow rates into a microreactor or packed bed column. After reaction was complted, the reaction mixture is collected at the outlet. Because of the inherent design of specific module, hazardous and explosive reaction are possible than batch reaction. It can continuously manufacture high quality API.
-